Breath analysis by MS is traditionally performed for small molecules (semi-volatile metabolites), which is an indirect approach producing highly complex spectra and with many open questions regarding the universal character of many such biomarkers. The analysis of intact viruses directly from breath in real-time poses completely different challenges and has never been accomplished so far,despite knowing that the breath of patients is a rich source of viral particles, which are responsible for the dispersion of the disease. Provided that new technology is designed and tailored to this application, with the exciting expansion to bacteria and cells, it is envisaged that real-time screening of the population by measuring intact viruses directly from breath and in water, complemented by top-down information to distinguish between different viral strains (by sequencing glyco- or capsid proteins), including post-genetic and post-proteomic analysis of isolated virions, is a challenging but realistic endeavour that can be realized in the ARIADNE platform.
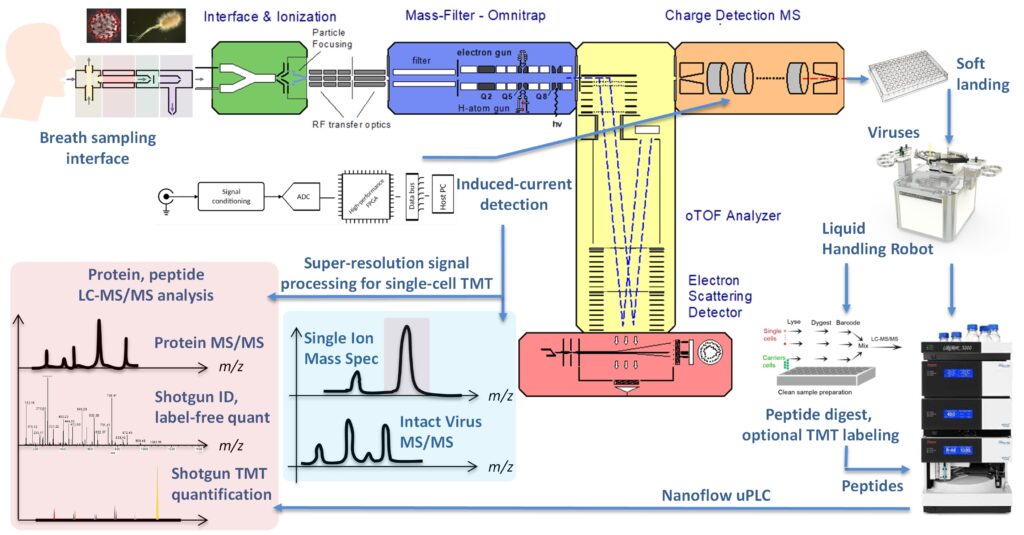
The diversified objectives of ARIADNE can be categorized as follows. (a) To provide fast, real-time, highly accurate monitoring of intact viruses in breath. The new design will be addressing limitations in existing technology extending from the sampling stage, to particle focusing, transportation, storage, processing and subsequent detection of these species. Successful implementation requires generating a versatile, gas-dynamically optimized sampling system capable of pre-concentrating viruses sampled from breath with direct injection to the mass spectrometer and post-ionization in mixed laminarized low pressure subsonic flows and/or post-collection and subsequent dispersion into the gas phase using Electrospray Ionization (ESI). The ESI interface will also allow for analysing water-based samples. (b) To characterize and classify viruses by utilizing a novel TOF analyser equipped with an electron scattering detector (eTOF). The performance of conventional electron multipliers is limited as the mass of the analyte increases, while in contrast, in the new design the detection efficiency is expected to increase with mass. This reversed property of the new design eliminates a fundamental limitation inherent to existing TOF systems with a great potential of extending the applications of MS to the analysis of intact particulate matter. (c) To perform tandem MSn of viruses in the Omnitrap platform for high level of identification. This unique technology is perfectly suited for top-down and complex-down analysis of viral particles, owing to the rectangular RF technology for trapping and processing charged particles in the “real” ultra-high mass range. High energy protons, laser light and high energy collisions will be deployed for dissociating surface proteins, enriching their population by operating the Omnitrap in accumulation mode and ultimately, subjecting these proteins to additional stages of fragmentation involving electrons. These multistage MS capabilities can be used for sequencing and unparalleled identification of virus variants. (d) To measure individual highly charged particles in a non-destructive manner. A cutting-edge FT-CDMS will be integrated and will operate in both high-throughput (single pass) and in high-resolution (trapping) modes. In the single-pass mode a novel arrangement of induced-current detection-electrodes will be employed to produce a mass spectrum. In ion trapping mode, charged particles are trapped between two mirrors and induced-current is observed over a prolonged period of time for enhanced sensitivity, resolving power and accuracy. In addition, photo-fragmentation reactions induced by an infrared laser will be performed. The objective is to establish a link between the photo-fragmentation observed in viruses and their structure, providing yet another level of identification. These efforts will be supported with advanced signal acquisition electronics with the new-generation architecture and real-time digital signal processing. (e) To recover soft-landed mass selected charged particles for single-cell proteomics workflows. Proteomics of the collected individual viruses, bacteria, cells will be performed by state-of-the-art Orbitrap and timsTOF MS. Analysing single human cells to the depth of >1000 proteins is shown to be feasible. Extending this work to viruses already measured by MS in a non-destructive manner will provide an unprecedented level of characterization and classification of these species.
